Supervisor: dr hab. Stefan Chłopicki
Jagiellonian Centre for Experimental Therapeutics
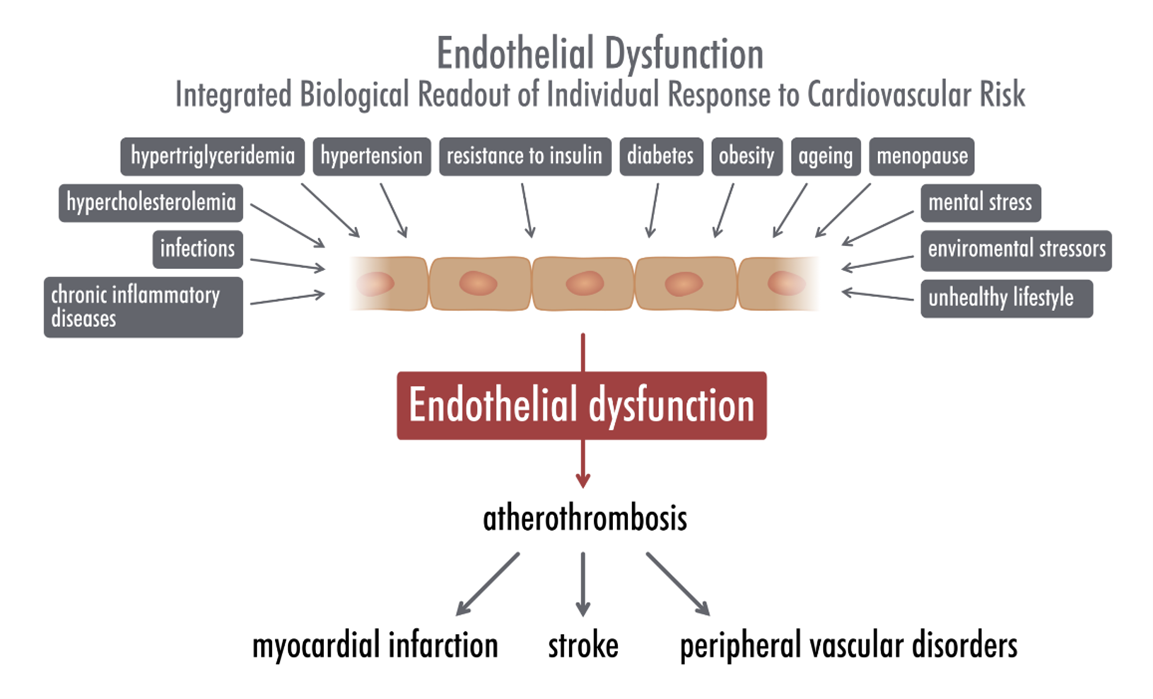 Chronic, sterile, low-grade inflammation observed in older organism that have been recently named “inflamm-ageing”, results in accelerated development of endothelial dysfunction and large arteries stiffness and can be associated with alterations in vascular mitochondrial function [1, 2]. Systemic endothelial dysfunction and increased stiffness of large arteries, can be measured in clinical conditions, and predict morbidity and mortality of cardiovascular diseases. Accordingly, the improvement in endothelial function and artery stiffness can have therapeutic effects. However, mechanisms involved in inflamm-ageing are not clear. We hypothesize that age-dependent dysfunctional vasculature in inflamm-ageing”, might be linked to vascular metabolic reprogramming that could contribute to vascular inflamm-ageing and subsequently to persistent vascular inflammation, to endothelial dysfunction and arterial stiffness. We aim to characterize metabolic signature of inflamm-ageing in murine models, and in particular to define the mechanisms and importance of metabolic reprogramming in the development of age-dependent endothelial dysfunction in large arteries and in coronary microcirculation, as well as in arterial stiffness. On the methodological level this research project is based on interdisciplinary, state-of-the art methodologies used in our recent studies [3-7] including e.g.; Magnetic Resonance Imaging – MRI to assess endothelial function in vivo in mice, vascular preparations for functional and metabolomic studies, as well as characterization of in vitro phenotype of primary endothelial cells isolated from mice. Targeted and non-targeted metabolomics will be used to define metabolic pathways of dysfunctional endothelium and vascular wall. The research is carried out in the frame of MAESTRO grant and international collaboration.
Chronic, sterile, low-grade inflammation observed in older organism that have been recently named “inflamm-ageing”, results in accelerated development of endothelial dysfunction and large arteries stiffness and can be associated with alterations in vascular mitochondrial function [1, 2]. Systemic endothelial dysfunction and increased stiffness of large arteries, can be measured in clinical conditions, and predict morbidity and mortality of cardiovascular diseases. Accordingly, the improvement in endothelial function and artery stiffness can have therapeutic effects. However, mechanisms involved in inflamm-ageing are not clear. We hypothesize that age-dependent dysfunctional vasculature in inflamm-ageing”, might be linked to vascular metabolic reprogramming that could contribute to vascular inflamm-ageing and subsequently to persistent vascular inflammation, to endothelial dysfunction and arterial stiffness. We aim to characterize metabolic signature of inflamm-ageing in murine models, and in particular to define the mechanisms and importance of metabolic reprogramming in the development of age-dependent endothelial dysfunction in large arteries and in coronary microcirculation, as well as in arterial stiffness. On the methodological level this research project is based on interdisciplinary, state-of-the art methodologies used in our recent studies [3-7] including e.g.; Magnetic Resonance Imaging – MRI to assess endothelial function in vivo in mice, vascular preparations for functional and metabolomic studies, as well as characterization of in vitro phenotype of primary endothelial cells isolated from mice. Targeted and non-targeted metabolomics will be used to define metabolic pathways of dysfunctional endothelium and vascular wall. The research is carried out in the frame of MAESTRO grant and international collaboration.
References:
[1] Liberale L, Montecucco F, Tardif JC, Libby P, & Camici GG (2020). Inflamm-ageing: the role of inflammation in age-dependent cardiovascular disease. Eur Heart J 41, 2974-2982. https://pubmed.ncbi.nlm.nih.gov/32006431/
[2] Tyrrell DJ, Blin MG, Song J, Wood SC, & Goldstein DR (2020). Aging Impairs Mitochondrial Function and Mitophagy and Elevates Interleukin 6 Within the Cerebral Vasculature. J Am Heart Assoc 9, e017820. https://pubmed.ncbi.nlm.nih.gov/33225820/
[3] Bar A, Targosz-Korecka M, Suraj J, Proniewski B, Jasztal A, Marczyk B, et al. Degradation of Glycocalyx and Multiple Manifestations of Endothelial Dysfunction Coincide in the Early Phase of Endothelial Dysfunction Before Atherosclerotic Plaque Development in Apolipoprotein E/Low-Density Lipoprotein Receptor-Deficient Mice. J Am Heart Assoc. 2019;8(6):e011171. https://pubmed.ncbi.nlm.nih.gov/30866689/
[4] Mohaissen T, Proniewski B, Targosz-Korecka M, Bar A, Kij A, Bulat K, et al. Temporal relationship between systemic endothelial dysfunction and alterations in erythrocyte function in a murine model of chronic heart failure. Cardiovasc Res. 2022;118(12):2610-24. https://pubmed.ncbi.nlm.nih.gov/34617995/
[5] Karas A, Bar A, Pandian K, Jasztal A, Kurylowicz Z, Kutryb-Zajac B, et al. Functional deterioration of vascular mitochondrial and glycolytic capacity in the aortic rings of aged mice. Geroscience. 2024. https://pubmed.ncbi.nlm.nih.gov/38418756/
[6] Pospiech E, Bar A, Pisarek-Pacek A, Karas A, Branicki W, Chlopicki S. Epigenetic clock in the aorta and age-related endothelial dysfunction in mice. Geroscience. 2024. https://pubmed.ncbi.nlm.nih.gov/38381284/
[7] Wojnar-Lason K, Tyrankiewicz U, Kij A, Kurpinska A, Kaczara P, Kwiatkowski G, et al. Chronic heart failure induces early defenestration of liver sinusoidal endothelial cells (LSECs) in mice. Acta Physiol. 2024. https://pubmed.ncbi.nlm.nih.gov/38391060/


 Chronic, sterile, low-grade inflammation observed in older organism that have been recently named “inflamm-ageing”, results in accelerated development of endothelial dysfunction and large arteries stiffness and can be associated with alterations in vascular mitochondrial function [1, 2]. Systemic endothelial dysfunction and increased stiffness of large arteries, can be measured in clinical conditions, and predict morbidity and mortality of cardiovascular diseases. Accordingly, the improvement in endothelial function and artery stiffness can have therapeutic effects. However, mechanisms involved in inflamm-ageing are not clear. We hypothesize that age-dependent dysfunctional vasculature in inflamm-ageing”, might be linked to vascular metabolic reprogramming that could contribute to vascular inflamm-ageing and subsequently to persistent vascular inflammation, to endothelial dysfunction and arterial stiffness. We aim to characterize metabolic signature of inflamm-ageing in murine models, and in particular to define the mechanisms and importance of metabolic reprogramming in the development of age-dependent endothelial dysfunction in large arteries and in coronary microcirculation, as well as in arterial stiffness. On the methodological level this research project is based on interdisciplinary, state-of-the art methodologies used in our recent studies [3-7] including e.g.; Magnetic Resonance Imaging – MRI to assess endothelial function in vivo in mice, vascular preparations for functional and metabolomic studies, as well as characterization of in vitro phenotype of primary endothelial cells isolated from mice. Targeted and non-targeted metabolomics will be used to define metabolic pathways of dysfunctional endothelium and vascular wall. The research is carried out in the frame of MAESTRO grant and international collaboration.
Chronic, sterile, low-grade inflammation observed in older organism that have been recently named “inflamm-ageing”, results in accelerated development of endothelial dysfunction and large arteries stiffness and can be associated with alterations in vascular mitochondrial function [1, 2]. Systemic endothelial dysfunction and increased stiffness of large arteries, can be measured in clinical conditions, and predict morbidity and mortality of cardiovascular diseases. Accordingly, the improvement in endothelial function and artery stiffness can have therapeutic effects. However, mechanisms involved in inflamm-ageing are not clear. We hypothesize that age-dependent dysfunctional vasculature in inflamm-ageing”, might be linked to vascular metabolic reprogramming that could contribute to vascular inflamm-ageing and subsequently to persistent vascular inflammation, to endothelial dysfunction and arterial stiffness. We aim to characterize metabolic signature of inflamm-ageing in murine models, and in particular to define the mechanisms and importance of metabolic reprogramming in the development of age-dependent endothelial dysfunction in large arteries and in coronary microcirculation, as well as in arterial stiffness. On the methodological level this research project is based on interdisciplinary, state-of-the art methodologies used in our recent studies [3-7] including e.g.; Magnetic Resonance Imaging – MRI to assess endothelial function in vivo in mice, vascular preparations for functional and metabolomic studies, as well as characterization of in vitro phenotype of primary endothelial cells isolated from mice. Targeted and non-targeted metabolomics will be used to define metabolic pathways of dysfunctional endothelium and vascular wall. The research is carried out in the frame of MAESTRO grant and international collaboration.